Former Food and Drug Administration (FDA) Commissioner Scott Gottlieb is questioning FDA Commissioner Stephen Hahn’s comments about fast-tracking a coronavirus vaccine, stating that a full approval of a vaccine for the general population likely won’t happen until 2021.
Hahn said in an interview published by The Financial Times today that he is willing to fast-track a coronavirus vaccine before clinical trials are complete if it is determined to be “appropriate.”
“I don’t know what is meant by saying before the phase three trials are completed,” Gottlieb said later on CBS’s “Face the Nation,” in response to Hahn’s interview.
“These phase three trials are event-based trials, meaning that they’re going to start to read out data after a certain amount of events accrue in the clinical trials. And those events are people getting COVID infection. And so as the trials progress, if we start to see lower rates of COVID infection in the active group, the group that receives the vaccine versus the placebo group, the group that hasn’t received the vaccine,” Gottlieb added.
He said the trials could read out data earlier, in October, if the vaccines are “very effective.”
“And so I’m not sure what he means by approving it earlier than when the trials are completed. They’re going to wait for these trials to read out before they can make a decision around the efficacy of these vaccines,” Gottlieb said.
“I think, again, of full approval for the general population, where people can go to CVS and get a shot, that’s really a 2021 event, maybe the first quarter of 2021, probably more likely the first half,” he added.
Hahn told the Financial Times that politics would play no part in any decision to fast-track a coronavirus vaccine.
“It is up to the sponsor [vaccine developer] to apply for authorization or approval, and we make an adjudication of their application,” Hahn said. “If they do that before the end of Phase Three, we may find that appropriate. We may find that inappropriate, we will make a determination.”
Gottlieb also responded to the new guidance issued by the Centers for Disease Control and Prevention this week that said people who have been exposed to the coronavirus but remain asymptomatic may not need to be tested.
“We should be testing those people to make sure they haven’t become infected and aren’t asymptomatic carriers because we know that they can spread the infection,” he said. “They’re less likely to spread the infection, but they can still spread the infection.”
Gottlieb said he learned one motivation behind the new guidance was a requirement from some businesses that workers test negative before they can return to work.
“If that’s the case and that was a concern, there were more targeted ways to address that and speak to that problem, as opposed to making this very broad, sweeping change in the recommendations, which I think could be misinterpreted by the general public and certainly by public health agencies within states,” he said. “And so I don’t think this changed guidance is likely to be followed by many states. I think it’s prudent that we test people who might be at high risk of contracting the infection.”
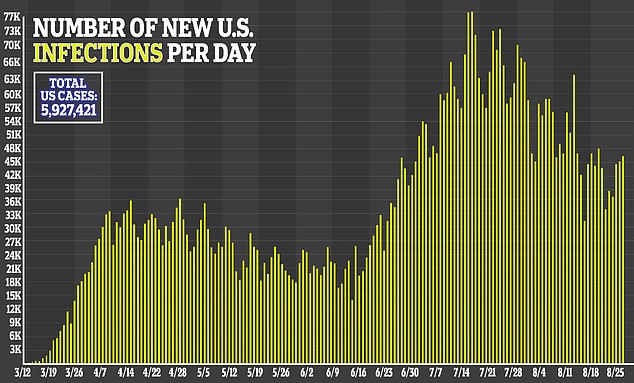
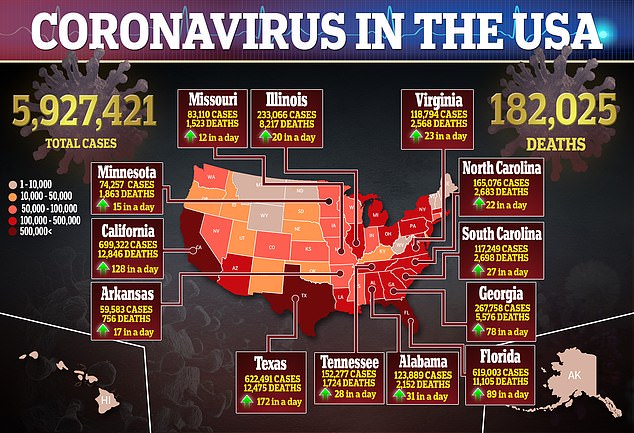
Drug manufacturers around the world are working to develop a coronavirus vaccine. The virus has infected more than 25 million people and resulted in more than 843,000 worldwide, according to data compiled by Johns Hopkins University.